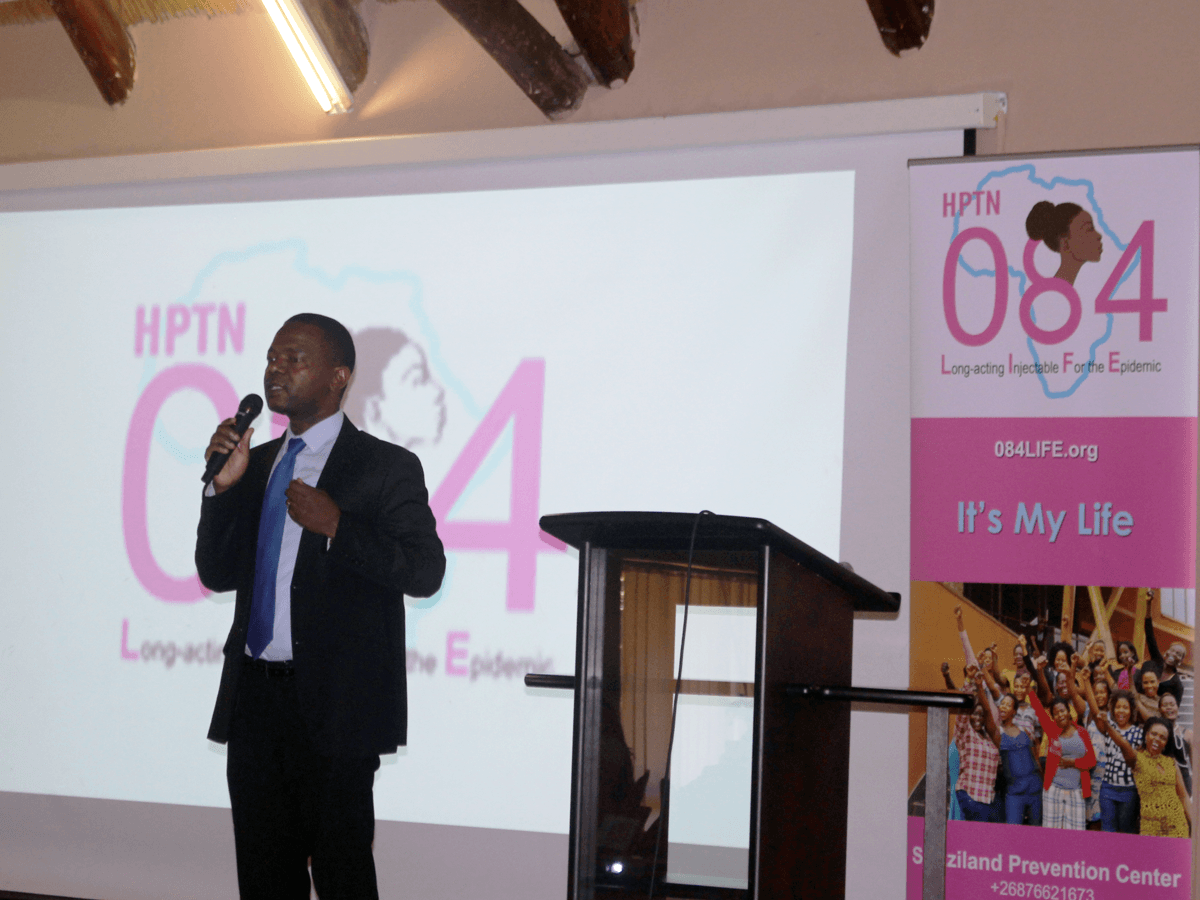
Representatives from the Eswatini Ministry of Health on October 25, 2018, officially launched a groundbreaking study of a new, long-acting, injectable drug for the prevention of HIV infection. This is the first investigational drug trial testing a new form of pre-exposure prophylaxis (PrEP) to prevent HIV infection. The HIV Prevention Trials Network (HPTN) study, called HPTN 084 or the LIFE (Long-acting Injectable For the Epidemic) study, will evaluate the safety and efficacy of the new injectable drug, cabotegravir, when compared to oral PrEP using Truvada® among HIV-negative women.
The availability of a long-acting form of PrEP given by injection every two months would overcome some of the barriers to uptake and adherence associated with having to take pills every day, as is currently the need with oral PrEP. These barriers include the need to store the pills at home in a safe location, and fear that a family member or sexual partner may notice or find the pills.
In Eswatini, despite impressive success in reducing the rate of new HIV infections, the country continues to contend with a severe HIV epidemic. Njabuliso Lukhele, MD, Senior Medical Officer for Public Health and the Eswatini Ministry of Health lead investigator on the study, welcomed the opportunity to participate in HPTN 084, hailing this as a critical advance for national capacity building while contributing to the global research agenda to find new HIV prevention methods. “Randomized controlled trials are important in testing new treatment, prevention, and diagnostic products, providing data on how safe a product is, how well it works, and how participants tolerate it,” he said.
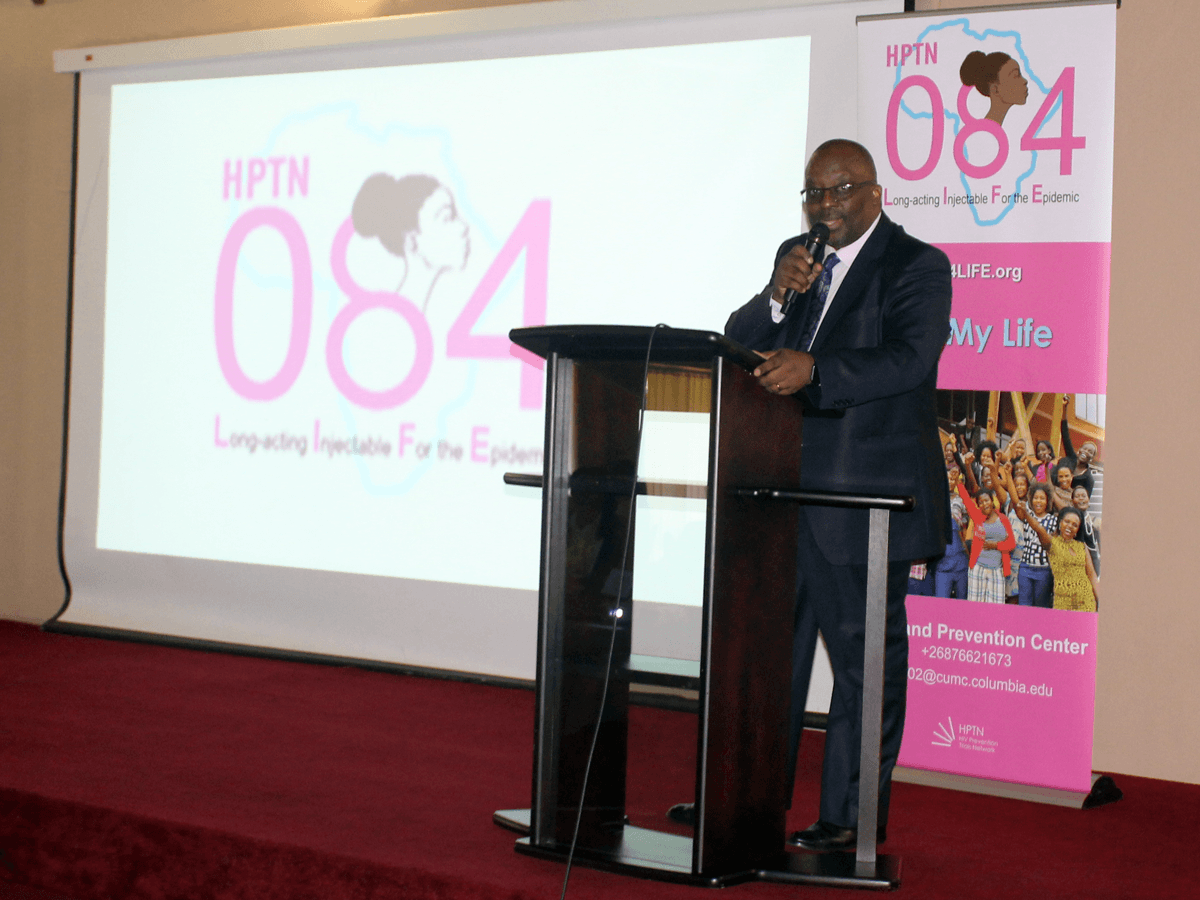
More than a year of intensive collaborative effort between the ICAP in Eswatini team, the Eswatini Ministry of Health, the Family Life Association of Eswatini (FLAS), and HPTN has gone into building, equipping, and ensuring the proper accreditation for the site, as well as ensuring staff readiness, to implement this study. The site is Eswatini’s first research site accredited by the U.S. National Institutes of Health Division of AIDS (DAIDS) for clinical trials.
ICAP supports two other HIV prevention centers, both in the United States: Harlem Prevention Center and Bronx Prevention Center in New York City run the HPTN 083 study, a sister study that is evaluating the same injectable drug for PrEP among men who have sex with men and transgender women.
HPTN 084 aims to enroll 3,200 HIV-negative women from Botswana, Eswatini, Kenya, Malawi, South Africa, Uganda, and Zimbabwe. In Eswatini, the trial is overseen by the Ministry of Health in partnership with ICAP in Eswatini and FLAS. Harriet Nuwagaba-Biribonwoha, MD, PhD, research director for ICAP in Eswatini, is the principal investigator of HPTN 084 in Eswatini.
“This is a significant milestone in the ongoing efforts to enhance research capacity in Eswatini,” said Dr. Nuwagaba-Biribonwoha, who is also principal investigator of the Health Research Training Program for young researchers in Eswatini. “We are proud to collaborate with HPTN and contribute to this large-scale, critically important study on HIV prevention.“

ICAP’s founder and global director, Wafaa El-Sadr, MD, MPH, MPA, is co-principal investigator of HPTN as a whole, along with Myron S. Cohen, MD, from the University of North Carolina at Chapel Hill. HPTN is funded by the U.S. National Institutes of Health.
“Finding new ways to prevent the spread of HIV is critically important,” Dr. El-Sadr said. “It is particularly exciting to have Eswatini join the HPTN 084 study and we look forward to its many contributions to this effort.”
View the “HPTN 084 and Enhancing the PrEP Toolbox for Women” video here
(Times of Swaziland) HPTN 084 study to enroll 148 HIV-negative women for HIV prevention trial
(Swazi Observer) HPTN study an opportunity for Eswatini to contribute on PrEP
Pictures are from the launch of the HPTN 084 study in Eswatini on October 25, 2018. Header image features Dr. Simon Zwane, Principal Secretary of the Ministry of Health, delivering remarks at the launch event. Second image features MOH Deputy Director Dr. Vusi Magagula introducing Dr. Zwane. Third image features Dr. Zwane officially handing over the HPTN 084 approval documents to symbolize commissioning of the study; left to right the presenters are: Ms. Zandile Mnisi, Eswatini Ministry of Health, Mr. Bongani Msibi, FLAS, Dr. Harriet Nuwagaba-Biribonwoha, ICAP in Eswatini, Ms. Nomsa Shongwe, Eswastini Ministry of Health, Dr. Ruben Sahabo, ICAP in Eswatini, Dr. Njabulo Lukhele, Eswatini Ministry of Health, and Dr. Simon Zwane, Eswatini Ministry of Health.