United States
United States
While ICAP is renowned for its work supporting health in countries around the world, it also carries out vital initiatives in New York City that serve key communities and inform new approaches to global health.
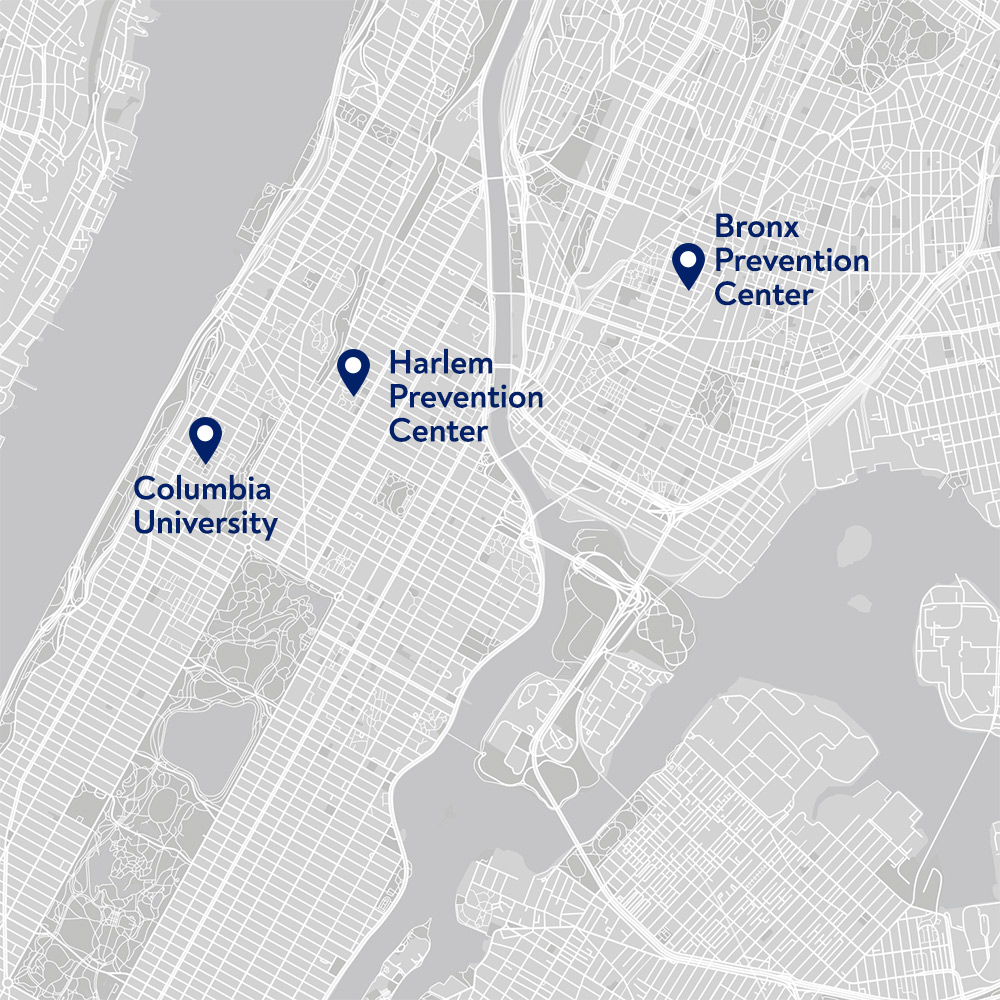
From its headquarters at the Mailman School of Public Health, which is part of the Columbia University Irving Medical Center, ICAP draws on world-class resources and expertise to conduct research and design programs that transforms the health of people worldwide.
ICAP’s two research centers in New York City, the Bronx Prevention Center and the Harlem Prevention Center, carry out cutting-edge biomedical and public health investigations in areas that have been historically underserved and disproportionately impacted by HIV and, more recently, COVID-19 and mpox. ICAP works with local community-based organizations, religious leaders, and advocates to promote community awareness of and engagement with the research studies.
Through Columbia University, ICAP also supports additional COVID-19 surveys and various student fellowship programs, engaging students to participate in the design, implementation, and evaluation of ICAP-supported programs while working side-by-side with its experts.

Projects

ACTG 5418 STOMP Study
- current: Harlem
DMID 19-0004 (The MAGI Study)
- current: Harlem
HPTN 091: I AM Study
- current: Harlem
HPTN 094: The INTEGRA Study
- current: Bronx
HTPN 083: Give PrEP a Shot
- current: Bronx, Harlem
Making PrEP Smart
- current: Bronx
The LEXICON Study 2.0
- current: United States
Clinical Trials
ICAP at Columbia University Clinical Trials Unit (ICAP CTU)
An innovative and comprehensive approach to HIV prevention and therapeutic research.
The ICAP CTU aims to support the design and implementation of research to address gaps in the global HIV response, including through clinical research sites in Harlem, Bronx, and Eswatini. Led by Wafaa El-Sadr, MD, MPH, MPA, and Jessica Justman, MD, this work is carried out through the ICAP at Columbia University Clinical Trials Unit (CTU).
ICAP in the United States
Active Years
2003 to present
Key Technical Areas
- Clinical Research and Trials
- COVID-19 Research
- PrEP Research
- Surveys
- Sexually Transmitted Infections Research
Current Funders
- The Rockefeller Foundation
- The Samuels Foundation
- The New York Community Trust
ICAP Founder and Global Director
 Wafaa El-Sadr, MD, MPH, MPA
Wafaa El-Sadr, MD, MPH, MPA
Dr. Wafaa El-Sadr is the founder and director of ICAP and an international expert in epidemiology and research on the prevention and management of HIV, tuberculosis, and other infectious diseases. She is also the director of Columbia World Projects. For over three decades, she has advocated for families and communities most impacted by HIV and championed a collaborative, multidisciplinary approach to confronting the global epidemic.
Based at Columbia University, she leads ICAP’s portfolio of projects in 30 countries and manages a global team of over 2,000 staff. Under her leadership, ICAP has become a global leader in HIV and health systems strengthening. She is also the director of the Mailman School’s Global Health Initiative (GHI), which mobilizes the University community to address critical challenges in global health.

The New York City Pandemic Response Institute (PRI)
Prepare.Recover.Invest
PRI is a landmark initiative designed to help prepare NYC for future public health threats – from infectious diseases to climate-related health emergencies – by fortifying and diversifying the city’s public health infrastructure, improving health equity, and elevating NYC as a model of public health preparedness across the globe.
Jobs in the United States
See the ICAP careers page to search all job listings.