Harlem
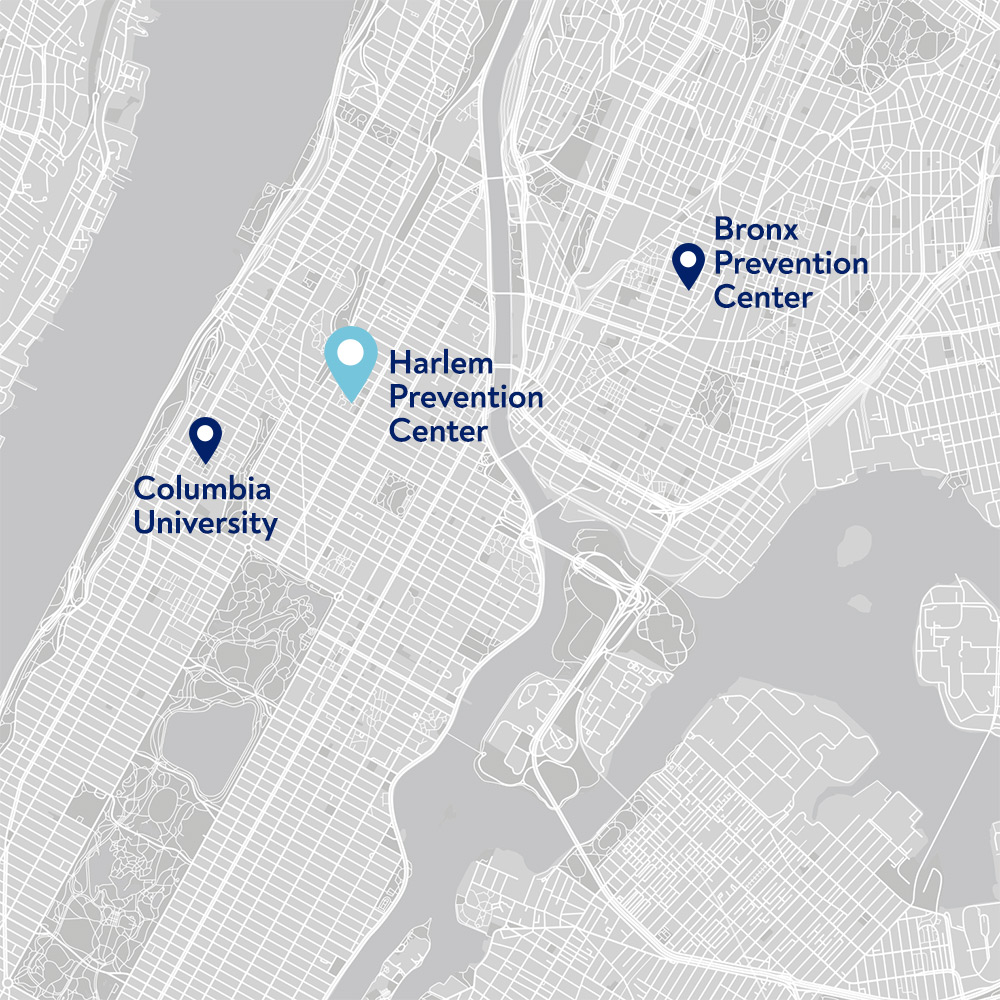
Harlem Prevention Center
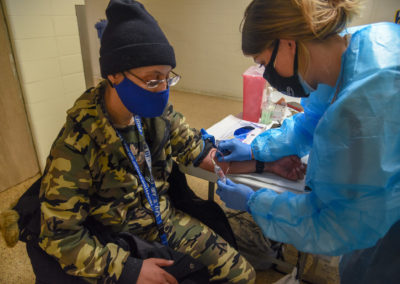
The Harlem Prevention Center was launched in 2009 as an HIV Prevention Trials Network (HPTN) clinical research site to advance research in HIV prevention, care, and treatment, focusing on underrepresented and medically underserved communities in New York City.
The site, as part of ICAP’s Clinical Trials Unit, has successfully participated in several HPTN studies, including HPTN 061 (The BROTHERS Study), a multi-site acceptability and feasibility study of HIV and STI testing, HIV risk reduction counseling, condom distribution, and peer navigation for HIV risk reduction; HPTN 064 (ISIS Study), a venue–based recruitment in neighborhoods with high rates of HIV and poverty conducted in six US cities; and HPTN 067 (ADAPT Study) where Harlem Prevention Center was the only US site chosen to implement this Pre-Exposure Prophylaxis (PrEP) trial, investigating the feasibility and acceptability of alternative PrEP dosing with three distinct populations in Bangkok, Cape Town and Harlem.
Current studies include investigations of antibody-mediated prevention using broadly neutralizing antibodies (bnAbs) to prevent HIV or COVID-19, an injectable long-acting pre-exposure prophylaxis (PrEP) option, mobile technologies to improve PrEP adherence, integrated care for people who inject opiates, HIV vaccine studies, and studies of COVID-19 immunity, vaccine efficacy, and treatment among others.


Projects

ACTG 5418 STOMP Study
- current
DMID 19-0004 (The MAGI Study)
- current
HPTN 091: I AM Study
- current
HTPN 083: Give PrEP a Shot
- current
ICAP in Harlem
Active Years
2009 to Present
Key Technical Areas
- Clinical Research and Trials
- COVID-19 Research
- PreP Research
- Surveys
Current Funders
- National Institute of Health
- National Institute of Allergy and Infectious Diseases (NIH/NIAID)
- Department of Health and Human Services (DHHS)
- Family Health International (FHI)
- Fred Hutchinson Cancer Research Center
- Janssen
Site Leader
Yael Hirsch-Moverman, PhD, MPH
Dr. Hirsch-Moverman is an epidemiologist with over 20 years of experience in epidemiologic and implementation research in TB and HIV. Her deep immersion in TB and HIV prevention and treatment research began in New York City, investigating and comparing different approaches to increase adherence to medications among underserved populations. Ultimately, her focus broadened to Africa, where an explosion of TB cases has overlapped with the HIV epidemic.
Dr. Hirsch-Moverman’s work has spanned biomedical domains, including epidemiology and clinical trials, and behavioral domains, including psychosocial issues such as adherence. She is currently focused on designing, conducting, and analyzing the effectiveness and acceptability of interventions to improve patient-centered care in TB and HIV programs in resource-limited settings where TB/HIV infection rates are high. She supports and leads several implementation science research studies that focus on developing evidence-based novel approaches for TB prevention among children in resource-limited settings.
Another aspect of her work focuses on the prevention of HIV in vulnerable populations in the US, namely men who have sex with men (MSM) and transgender women (TGW). She is based at the Harlem Prevention Center Clinical Research Site (CRS), which NIAID funds to conduct HIV prevention and COVID-19 prevention research studies. In addition, the center conducts investigator-initiated research studies examining interventions that increase access to HIV testing and pre-exposure prophylaxis among MSM and TGW.
Jobs in the United States
See the ICAP careers page to search all job listings.