Clinical Trials
ICAP at Columbia University Clinical Trials Unit (ICAP CTU)
An innovative and comprehensive approach to HIV prevention and therapeutic research.
Led by Wafaa El-Sadr, MD, MPH, MPA and Jessica Justman, MD, ICAP CTU aims to support the design and implementation of research to address gaps in the global HIV response, including through clinical research sites in the Harlem, Bronx, and Eswatini.
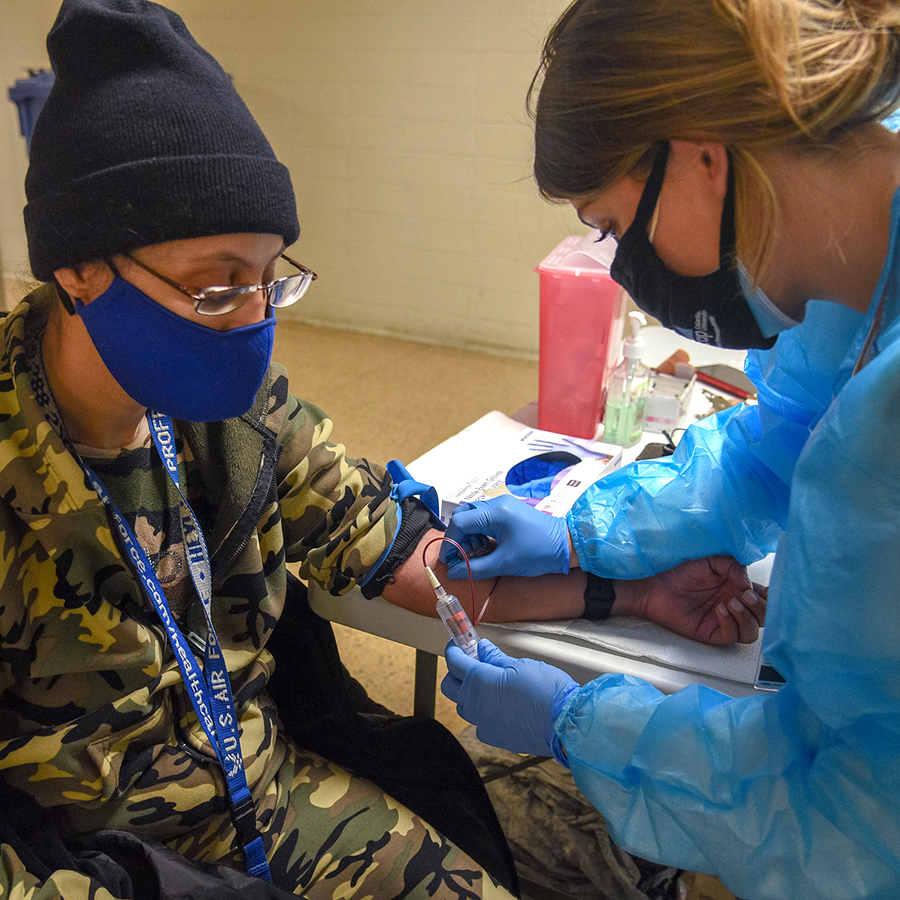
The Bronx and Harlem Prevention Centers
ICAP’s two research centers in New York City, the Bronx Prevention Center, and the Harlem Prevention Center, are hubs of research that have been historically underserved and disproportionately impacted by the HIV epidemic and, more recently, the COVID-19 epidemic. ICAP works with local community-based organizations, religious leaders, and advocates to promote community awareness of and engagement with research studies.
The Community Advisory Board (CAB) for the CTU provides guidance and feedback on proposed studies, outreach strategies, study materials, and information regarding community perceptions of the potential or current research studies.
Eswatini
In Eswatini, ICAP, in collaboration with the Eswatini Ministry of Health and Family Life Association of Eswatini, established the Eswatini Prevention Center Clinical Research Site (Eswatini CRS). This pioneer CRS has the capacity to conduct clinical trials with investigational drugs and registered products. The clinical research is implemented in close collaboration with local stakeholders to strengthen clinical research systems and conduct studies that impact local and global public health programs. Over 2,400 participants from diverse populations have been enrolled in CRS studies and participated in studies relating to HIV prevention with oral and injectable pre-exposure prophylaxis (PrEP), COVID-19 vaccination, and HPV vaccination.
Bronx

HPTN 094: The INTEGRA Study
- current
HTPN 083: Give PrEP a Shot
- current
Making PrEP Smart
- current
Harlem

ACTG 5418 STOMP Study
- current
DMID 19-0004 (The MAGI Study)
- current
HPTN 091: I AM Study
- current
HTPN 083: Give PrEP a Shot
Eswatini

CoVPN 3008: UBUNTU Study
- current
HPTN 084 OLE Study
- current
HPV Vaccine Study
- current






