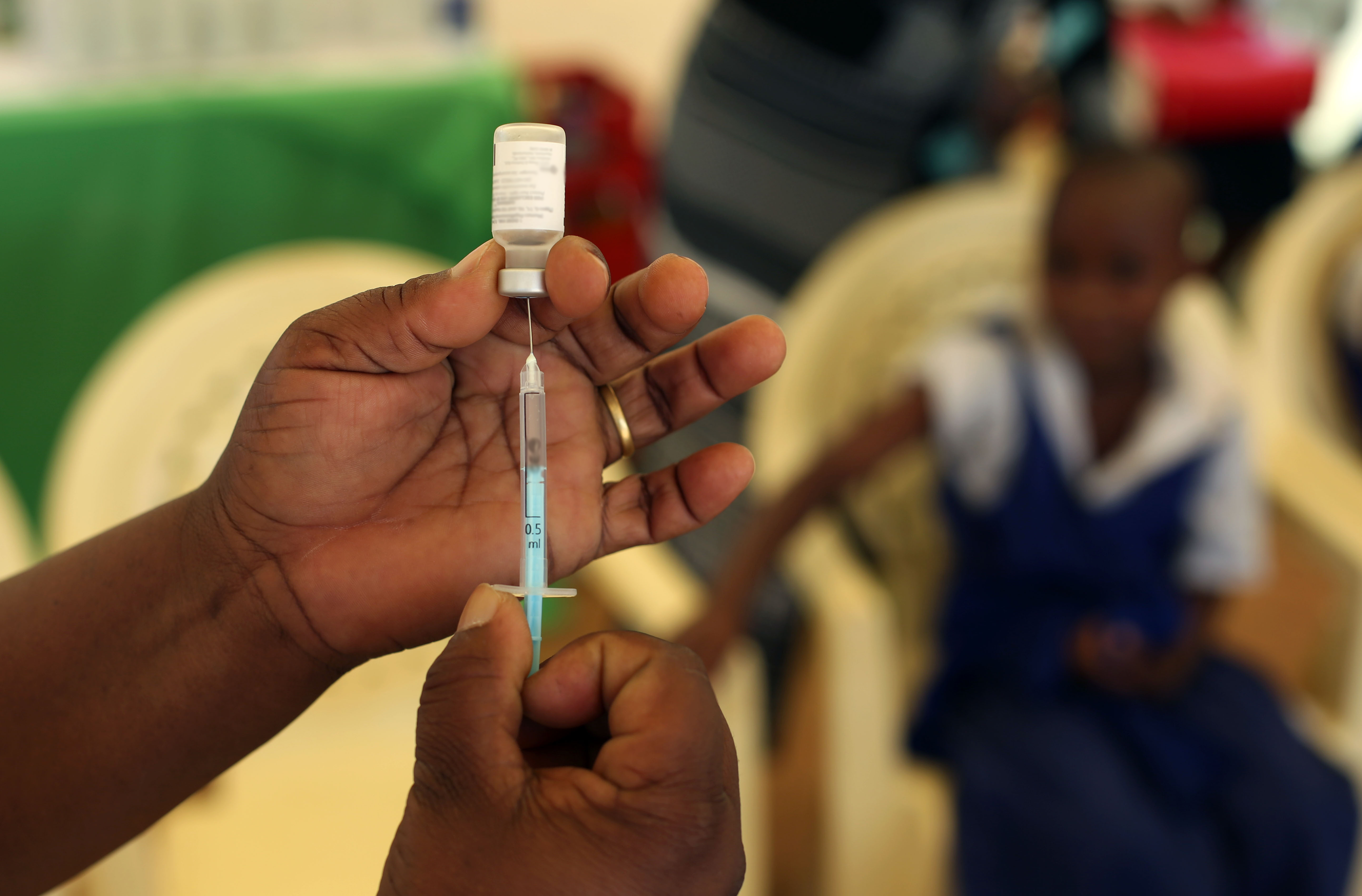
In Eswatini, ICAP at Columbia University, in partnership with the Ministry of Health (MOH) and other collaborators, has successfully enrolled 1,400 participants in a study assessing human papillomavirus (HPV) vaccine regimens for children, adolescents, and young women living with HIV. The study is critical to informing understanding of how to best introduce this highly effective vaccine to people living with HIV – a population six times more likely to develop pre-cancerous and cancerous cervical lesions after acquiring HPV infection compared to people not living with HIV.
Cervical cancer remains a significant threat to public health worldwide and is the leading cause of cancer-associated mortality in sub-Saharan African women. Nearly all cases of cervical cancer deaths are caused by HPV, which can be prevented by a vaccine that is 95 percent effective for women with a normal immune response. While the safety and efficacy of the HPV vaccine is well documented among healthy individuals, additional research on the HPV vaccine for people living with HIV is needed.
In Eswatini, cervical cancer is the leading malignancy in women of reproductive age and the leading cause of death in women 15-44 years of age. The added burden of HIV among young women in Eswatini, where HIV prevalence exceeds 30 percent in the reproductive age group, poses a serious public health concern.
The study was conducted in partnership with the Eswatini MOH, Baylor College of Medicine Children’s Foundation, Eswatini, The Bristol Myers Squibb Foundation, Merck Sharp & Dohme LLC, and the Reaching Impact, Saturation and Epidemic Control (RISE) consortium. Participant enrollment began in March 2022 and the study will compare a two-dose HPV vaccine series among children (9-14 years) and young women (15-26 years) living with HIV with a three-dose HPV vaccine series among HIV-uninfected young women (15-26 years). Data on immunogenicity – or the measurement of immune response – and safety of the two-dose regimen among people living with HIV is limited. This study is essential to provide data for this vulnerable population, especially young girls who are acquiring HPV infection and subsequently developing cervical cancer.
With a highly experienced and dedicated in-country ICAP study team, recruitment targets were quickly achieved, and enrollment was completed in September 2022. This rapid pace of enrollment was an important milestone given the challenges of the COVID-19 pandemic and reflects the high interest in the benefits of the HPV vaccine.
“It is great to see the young participants welcoming the opportunity to receive the HPV vaccine and eager to join the fight against cervical cancer,” said Harriet Nuwagaba-Biribonwoha, MD, PhD, research director of ICAP in Eswatini. “We look forward to seeing the results of the study and disseminating widely.”
“When I first heard about the HPV vaccine study, I was quite excited and encouraged my patients to participate because HPV can cause cervical cancer,” said Dr. Siphiwo Dube, Baylor COE Mbabane.
The study team has now pivoted to follow-up activities and will be tracking all enrolled participants for approximately one year, during which the study team will collect biodata, monitor adverse events, and document vaccination completion rates. The study is expected to be completed by September 2023.
This work is funded by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) Office of the U.S. Global AIDS Coordinator and Health Diplomacy (OGAC) through the U.S. Agency for International Development (USAID)-funded RISE consortium led by Jhpiego, with vaccine and immunogenicity testing provided by Merck Sharp & Dohme LLC.
“Launching this study was truly a team effort involving collaboration among multiple stakeholders,” said Xolisile Dlamini, lead investigator and Eswatini MOH lead for the National Cancer Control Program.
This study is providing the potentially lifesaving HPV vaccine for many individuals who do not currently have access to it. While plans for a nationwide rollout of the HPV vaccine are in progress, the vaccine is not currently available in public health care facilities across Eswatini. Global supply limitations and high costs have further hindered access to the vaccine in Eswatini, complicating the path to achieve optimal vaccination coverage.
“We have the tools to prevent cancer caused by HPV with vaccination, screening, and treatment,” said Elaine Abrams, MD, senior research director at ICAP. “By supporting this study, the Ministry of Health is taking another step towards the elimination of cervical cancer in Eswatini, and ensuring the vaccine reaches some of the most vulnerable populations.”
About ICAP
A major global health organization that has been improving public health in countries around the world for nearly two decades, ICAP works to transform the health of populations through innovation, science, and global collaboration. Based at Columbia Mailman School of Public Health, ICAP has projects in nearly 40 countries, working side-by-side with ministries of health and local governmental, non-governmental, academic, and community partners to confront some of the world’s greatest health challenges. Through evidence-informed programs, meaningful research, tailored technical assistance, effective training and education programs, and rigorous surveillance to measure and evaluate the impact of public health interventions, ICAP aims to realize a global vision of healthy people, empowered communities, and thriving societies.
About Reaching Impact Saturation and Epidemic Control (RISE)
RISE, a 5-year global project led by Jhpiego and funded by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) and the U.S. Agency for International Development (USAID), works with countries to achieve a shared vision of attaining and maintaining HIV epidemic control, with stronger local partners capable of managing and achieving results through sustainable, self-reliant, and resilient health systems by 2024. RISE’s contributions to this work will lead to fewer HIV transmissions, decreased HIV-related morbidity and mortality, and increased quality of life for people living with HIV. RISE also supports COVID-19 emergency response efforts in multiple countries, working with Ministries of Health and USAID to provide technical assistance and service delivery support for COVID-19, and helping to mitigate the effects of the COVID-19 pandemic on HIV services.
This success story was made possible through the United States Agency for International Development-funded RISE program under the terms of the cooperative agreement 7200AA19CA00003. The contents are the responsibility of the RISE program and do not necessarily reflect the views of USAID or the United States Government.