Bronx
Bronx Prevention Center
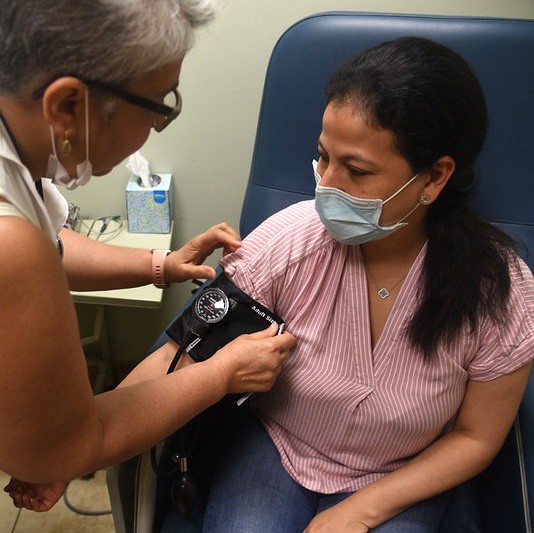
The Bronx Prevention Center (BPC) was established as part of ICAP’s Clinical Trials Unit to conduct HIV prevention studies in underrepresented and medically underserved communities in the South Bronx. Over the past decade, the BPC has established trust and engagement within the South Bronx community by designing and implementing complex and varied HIV prevention studies.
BPC has had great success enrolling underrepresented minorities at high risk of HIV acquisition in clinical trials, including men of color who have sex with men who have received little attention from researchers and public health programs in the past.
Current studies include investigations of antibody-mediated prevention using broadly neutralizing antibodies (bnAbs) to prevent HIV or COVID-19, an injectable long-acting pre-exposure prophylaxis (PrEP) option, mobile technologies to improve PrEP adherence, integrated care for people who inject opiates, HIV vaccine studies, and studies of COVID-19 immunity, vaccine efficacy, and treatment among others.

Projects


HPTN 094: The INTEGRA Study
- current
HTPN 083: Give PrEP a Shot
- current
Making PrEP Smart
- current
ICAP in Bronx
Active Years
2010 to present
Key Technical Areas
-
COVID-19
-
HIV
-
Sexually transmitted infections
-
Addiction
-
Health disparities
-
Stigma and Discrimination
Current Funders
- National Institute of Health
- National Institute of Allergy and Infectious Diseases (NIH/NIAID)
Department of Health and Human Services (DHHS) - Family Health International (FHI)
- Fred Hutchinson Cancer Research Center
- National Institute of Allergy and Infectious Diseases (NIH/NIAID)
- AstraZeneca
- Merck
- Gilead
Site Leader
 Ellen Morrison, MD, MPH
Ellen Morrison, MD, MPH
Dr. Ellen Morrison is the Site Leader of the Columbia University Mailman School of Public Health ICAP Bronx Prevention Center CRS. She is a practicing board-certified Infectious Disease physician who has been on the full-time faculty at the Columbia University Irving Medical Center for more than 30 years. Dr. Morrison has focused on clinical education, HIV testing and care, quality improvement, maternal infections, treatment of sexually transmitted infections, and the ethical review and conduct of clinical research. She joined ICAP in 2018, where she serves as a Senior Technical Specialist Advisor for ICAP’s global health initiatives and is an investigator and site lead at the Bronx Prevention Center. Dr. Morrison continues work on HIV prevention, including investigation of the provision of integrated services on a mobile van to engage out-of-care injection opiate users and work on COVID-19 vaccines, testing of monoclonal antibodies, and other COVID-19 treatment modalities.
Jobs in the United States
See the ICAP careers page to search all job listings.






